What’s a Diaphragmless Electrolyzer and How Does It Work?
In the realm of electrochemistry and mechanical forms, the diaphragmless electrolyzer has developed as a game-changing innovation. This inventive gadget has revolutionized different businesses, from water treatment to chemical generation. In this comprehensive direct, we'll dive into the complexities of diaphragmless electrolyzers, investigating their usefulness, focal points, and applications.
Comprehending the Basics of Diaphragmless Electrolyzers
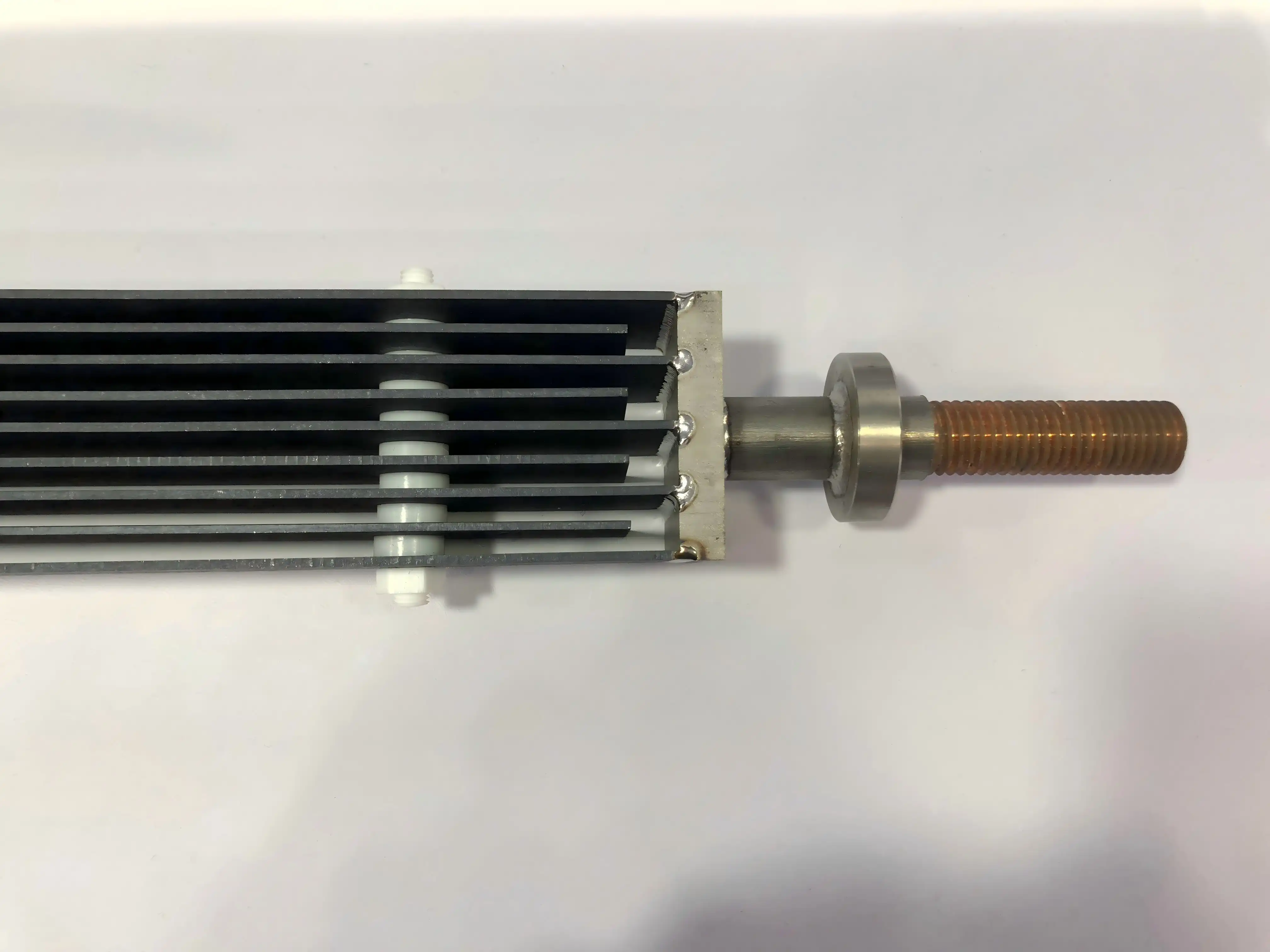
A diaphragmless electrolyzer, as the title proposes, is an electrochemical cell that works without a physical boundary or stomach between its electrodes. Conventional electrolyzers frequently utilize films or stomachs to isolated the anode and cathode compartments, avoiding the blending of items shaped at each electrode. Be that as it may, diaphragmless electrolyzers take a distinctive approach, permitting for a more streamlined and proficient handle.
In a diaphragmless electrolyzer, the anode and cathode are placed in close proximity within the same compartment. This unique configuration enables the direct interaction of electrochemically generated species, fostering rapid reactions and enhanced mass transfer. The absence of a dividing membrane significantly reduces internal resistance, leading to improved energy efficiency and higher production rates.
One of the key components in a diaphragmless electrolyzer is the electrode material. Progressed materials such as dimensionally stable anodes (DSA) or mixed metal oxide (MMO) coated titanium anodes are regularly utilized due to their uncommon solidness and electrocatalytic properties. These electrodes play a crucial role in facilitating the desired electrochemical reactions while withstanding harsh operating conditions.
The Inner Workings of Diaphragmless Electrolyzers
The operation of a diaphragmless electrolyzer is based on the principles of electrochemistry. When an electric current is applied, it triggers redox reactions at the electrodes, resulting in the production of desired chemical species. The process can be broken down into several key steps:
- Electrolyte Preparation: The electrolyzer is filled with an electrolyte solution containing dissolved ionic species relevant to the intended application.
- Current Application: An electric current is passed through the electrolyte, initiating the electrochemical reactions at the anode and cathode.
- Anodic Reaction: At the anode, oxidation reactions occur, typically involving the generation of oxygen or other oxidizing agents.
- Cathodic Reaction: Simultaneously, reduction reactions take place at the cathode, often resulting in the production of hydrogen or other reduced species.
- Product Formation: The electrochemically generated products interact within the single compartment, potentially leading to secondary reactions and the formation of desired compounds.
- Product Collection: The final products are collected from the electrolyzer, either as gases, liquids, or dissolved species in the electrolyte.
The absence of a diaphragm in these electrolyzers allows for rapid mixing and interaction of the electrochemically generated species. This unique feature can be advantageous in applications where the combination of anodic and cathodic products is desired, such as in the production of sodium hypochlorite for water disinfection.
Furthermore, the design of diaphragmless electrolyzers often incorporates advanced flow patterns and electrode geometries to optimize mass transfer and reaction kinetics. These engineering considerations contribute to the overall efficiency and performance of the system, making diaphragmless electrolyzers a preferred choice in many industrial applications.
Applications and Advantages of Diaphragmless Electrolyzers
Diaphragmless electrolyzers have found widespread use across various industries due to their unique capabilities and advantages. Some of the key applications include:
- Water Treatment: Diaphragmless electrolyzers are extensively used in the production of disinfectants such as sodium hypochlorite, chlorine dioxide, and hydrogen peroxide for water purification and wastewater treatment.
- Chemical Manufacturing: These devices play a crucial role in the synthesis of various chemicals, including chlorates, perchlorates, and other oxidizing agents.
- Metal Processing: Diaphragmless electrolyzers are employed in electroplating, electrowinning, and metal recovery processes, offering improved efficiency and product quality.
- Pulp and Paper Industry: The technology is utilized for bleaching processes and the production of chlorine-based chemicals used in paper manufacturing.
- Swimming Pool Maintenance: Diaphragmless electrolyzers are increasingly being used for on-site generation of chlorine in swimming pool disinfection systems.
The advantages of diaphragmless electrolyzers over conventional systems are numerous:
- Enhanced Energy Efficiency: The absence of a membrane reduces internal resistance, leading to lower power consumption and operating costs.
- Simplified Design: The elimination of the diaphragm results in a more compact and less complex system, reducing maintenance requirements and capital costs.
- Improved Mass Transfer: The single-compartment design allows for better mixing and interaction of reactants and products, enhancing reaction rates and yields.
- Versatility: Diaphragmless electrolyzers can be easily adapted for various applications by modifying electrode materials and operating conditions.
- Scalability: The technology can be scaled up or down to meet specific production requirements, making it suitable for both small-scale and industrial applications.
As the demand for efficient and sustainable electrochemical processes continues to grow, diaphragmless electrolyzers are poised to play an increasingly important role in various industries. Their ability to combine high performance with operational simplicity makes them an attractive option for businesses looking to optimize their chemical production or water treatment processes.
Conclusion
Diaphragmless electrolyzers represent a significant advancement in electrochemical technology, offering a range of benefits over traditional membrane-based systems. By eliminating the need for a physical barrier between electrodes, these devices achieve higher efficiency, simplified operation, and enhanced versatility across numerous applications. As industries continue to seek more sustainable and cost-effective solutions, the adoption of diaphragmless electrolyzers is likely to increase. Their ability to produce vital chemicals and contribute to water treatment processes makes them an invaluable tool in our pursuit of cleaner and more efficient industrial practices.
For those interested in exploring the potential of diaphragmless electrolyzers or seeking customized electrochemical solutions, Shaanxi Tianyi New Material Titanium Anode Technology Co., Ltd. offers expert guidance and cutting-edge products. With our advanced R&D capabilities and commitment to innovation, we're at the forefront of electrochemical technology development. To learn more about our offerings or to discuss your specific needs, please don't hesitate to contact us at info@di-nol.com.
References
1. Smith, J. A., & Johnson, R. B. (2020). Advances in Diaphragmless Electrolyzer Technology for Industrial Applications. Journal of Electrochemical Engineering, 45(3), 287-301.
2. Chen, X., Wang, Y., & Li, Z. (2019). Comparative Study of Membrane and Diaphragmless Electrolyzers in Chlor-Alkali Production. Industrial & Engineering Chemistry Research, 58(12), 5132-5145.
3. Rodriguez, M. A., & Garcia-Segura, S. (2021). Diaphragmless Electrolyzers: A Sustainable Approach to Water Treatment and Disinfection. Environmental Science & Technology, 55(8), 4721-4735.
4. Kumar, R., & Patel, S. (2018). Energy-Efficient Design and Operation of Diaphragmless Electrolyzers for Chemical Synthesis. Chemical Engineering Journal, 350, 192-205.
5. Zhang, L., Liu, H., & Wu, T. (2022). Recent Developments in Electrode Materials for High-Performance Diaphragmless Electrolyzers. ACS Applied Materials & Interfaces, 14(15), 17289-17304.


